How Greenhouse Gases Heat the Surface Revealed
October 18, 2020Over many years Eli, your humble Bunny, has explained things about the Greenhouse Effect in words that even Mom Rabett of blessed memory would understand. The Cyanobacteria's Friend would say, the secret is at the top of the atmosphere where the outgoing radiation to space must match the incoming from the sun and that means as greenhouse gases increase and the level of the atmosphere from which emission can reach space increases and colder, the surface must warm.
But how does the surface warm and in particular how does backradiation play a role. Thanks to Twitter, Eli has found a splendid argument which he will put into these margins. Might even get published.
Dear friends, let us start. We got the sun. Let's ignore the absorption of sunlight in the atmosphere, the Foote effect, and say that it all hits the ground and is absorbed
Greenhouse gases prevent IR radiation from the surface reaching space directly
The atmosphere is transparent in the visible, but, there are many regions of the IR where absorption is high, including those regions where CO2 and H2O absorb. A handy dandy number to carry about is that at the surface the average distance light can travel in the CO2 spectral region is 10 meters (or about 35 feet for you unethical customary unit users).
Energy does not stay in the molecule that absorbs the IR photon, to be re-radiated later. This is not so, it is quickly degenerated to thermal motion (translation, zipping about) via collisions. Thermalization requires about a 10 μs at atmospheric pressure. So where does the emission come from the bunnies ask?
Well, there is a considerable thermal energy at room temperature, and even much lower. True this average energy is low compared to even the lowest vibrational excitation of CO2 (which would be ~1000 K), but it is enough that a small, but significant fraction of CO2 molecules are always found in excited levels which can emit in the IR (about 6% at room temperature).
Eli has explained this many times before. The two new things are to recognize that
1. The amount of heat passing from one layer to the next has to be equal to the amount of heat absorbed from the sun and
2. the number of layers the heat must pass through depends on greenhouse gas concentration and ability to absorb IR. Another way of thinking about this is that the IR energy emitted from the surface has to undergo a number of absorption/emission cycles before reaching space.
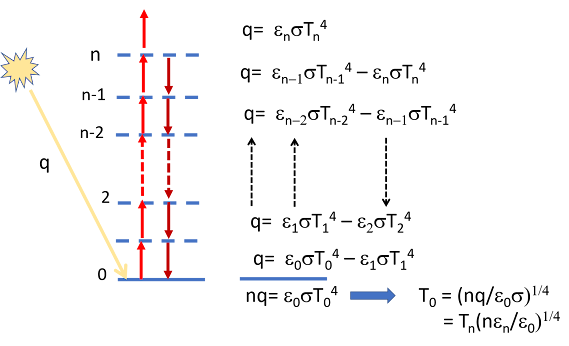
The figure below idealizes this showing that if there are effectively n layers each with an emissivity ε, that then the temperature at the bottom increases as the fourth root of n. when you add up the contribution of all the layers. We also know that the temperature of each layer decreases linearly with altitude for everything except water vapor where it decreases much faster because of condensation.
Remembering that q is both the heat absorbed from the sun and the heat radiated to space and substituting q= εnσTn⁴ where To is the temperature at the surface (say 287 K) and Tn the effective radiative temperature at the top (~255 K) we get n~ 4 after estimating that the altitude whose temperature is 255 K is between 5 and 6 km, where the density is approximately 40% of that at the surface.This is the mechanism by which the greenhouse effect warms the surface
If you are going to do a detailed calculation of the thermal structure of the atmosphere level by level, the separation between levels will depend on frequency as will the relative strength of different greenhouse gases. One of the tricky things is that greenhouse gases radiate at discrete fixed frequencies and the surface and clouds IR emit and absorb like black bodies, but more of that later.
So simply measuring the total absorption of a greenhouse gas at different frequencies in the atmosphere is not very useful.
Total absorption throughout the atmosphere does not account for the average distance that an IR photon can travel before being absorbed and thus the number of layers. For example, if the distance at 14 microns IR photon travels before being absorbed in 1% water vapor is 1 km, and that for 400 ppm CO2, 10 m, then CO2 would have much more of a warming effect on the surface at 14 μ but both would absorb 100% of the IR over the path from ground to space (and how).
Tomorrow, the concept being elastic and Eli tired, the Bunny will show how using the average distance that IR can travel at different frequencies helps understand the roles that different greenhouse gases play.