Rabett Run 2019-04-18 07:36:00
April 18, 2019
Yes, Eli has been on sabbatical, but he really has been thinking of the bunnies, especially with Easter coming up. Well, sometimes it just gets hard to think of something new and interesting and life, or at least Twitter, has become a series of re-runs where all that is needed is a link to some old Rabett Run post (there are some goodies there the Bunny will tell you:).
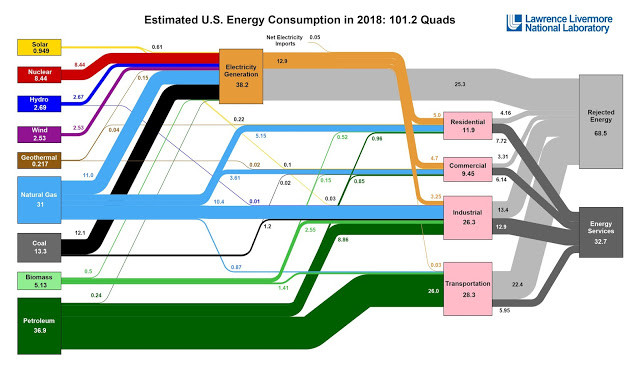
In any case there has been recent discussion about a chart posted by the Lawrence Livermore Lab folk about US Energy consumption and copied to Twitter. Several are trying to use this as an argument against fossil fuels pointing to the fact that rejected energy is a large part of electricity generation and more.
This is tied together with a basic confusion first discussed by RayP on Real Climate, that the problem with fossil fuels is not the rejected energy, but the fact that the greenhouse gas emissions remain in the atmosphere and slow the emission of heat to space over centuries. In a letter to Steve Levitt, Ray dispensed with Nathan Myhrvold's argument that
The reason that fossil fuels are heating the Earth is not that they produce heat as well as work, but that their CO2 emissions continue to increase the amount of thermal energy in the atmosphere, on the surface and in the oceans, over centuries.
Sunlight heats the Earth and is degraded to heat which radiates into space by IR emissions from the surface and the atmosphere. Increasing the amount of greenhouse gases in the atmosphere slows the rate of radiation so that the Earth has to warm, increasing the rate of radiation to return the system to balance. Energy added on the surface by fossil fuel combustion or nuclear energy or wind or solar, or whatever is soon degraded to thermal energy and radiated to space. It's a small one time charge.
The ratio between the work, W that can be done and the energy input U, W/U is the efficiency, and the heat produced is Q.
Thermodynamics sets the upper limit to efficiency. Engineering sets the actual limit. It turns out that renewables like wind and solar are not very efficient, but that the heat that they generate in creating work does not lead to a continual warming of the system over centuries. The same is true for heat generated in fossil fuel combustion, but, as RayP pointed out a decade ago, that is irrelevant, because the CO2 produced in fossil fuel combustion DOES heat the Earth for centuries.
That being said, it's good to be efficient. For one thing, it costs less over the long run in the building, operation and maintenance of stuff. Also, rejected heat is local, which can be both good and bad, depending if you can use the heat for other things like industrial processes, or heating the home. That gets deep into the weeds and Eli will leave it there
In any case there has been recent discussion about a chart posted by the Lawrence Livermore Lab folk about US Energy consumption and copied to Twitter. Several are trying to use this as an argument against fossil fuels pointing to the fact that rejected energy is a large part of electricity generation and more.
This is tied together with a basic confusion first discussed by RayP on Real Climate, that the problem with fossil fuels is not the rejected energy, but the fact that the greenhouse gas emissions remain in the atmosphere and slow the emission of heat to space over centuries. In a letter to Steve Levitt, Ray dispensed with Nathan Myhrvold's argument that
. . . in effect, that it was pointless to try to solve global warming by building solar cells, because they are black and absorb all the solar energy that hits them, but convert only some 12% to electricity while radiating the rest as heat, warming the planet. Now, maybe you were dazzled by Mr Myhrvold’s brilliance, but don’t we try to teach our students to think for themselves? Let’s go through the arithmetic step by step and see how it comes out. It’s not hard.Interested or, as in the case of Eli, those with the dread forgetting disease, can follow Ray through the calculation, but the point is that all energy eventually (may take the age of the universe, but eventually) degrades to heat, but some of it can be used in the meantime to do work (move things non-randomly, including electrons).
The reason that fossil fuels are heating the Earth is not that they produce heat as well as work, but that their CO2 emissions continue to increase the amount of thermal energy in the atmosphere, on the surface and in the oceans, over centuries.
Sunlight heats the Earth and is degraded to heat which radiates into space by IR emissions from the surface and the atmosphere. Increasing the amount of greenhouse gases in the atmosphere slows the rate of radiation so that the Earth has to warm, increasing the rate of radiation to return the system to balance. Energy added on the surface by fossil fuel combustion or nuclear energy or wind or solar, or whatever is soon degraded to thermal energy and radiated to space. It's a small one time charge.
The ratio between the work, W that can be done and the energy input U, W/U is the efficiency, and the heat produced is Q.
Thermodynamics sets the upper limit to efficiency. Engineering sets the actual limit. It turns out that renewables like wind and solar are not very efficient, but that the heat that they generate in creating work does not lead to a continual warming of the system over centuries. The same is true for heat generated in fossil fuel combustion, but, as RayP pointed out a decade ago, that is irrelevant, because the CO2 produced in fossil fuel combustion DOES heat the Earth for centuries.
That being said, it's good to be efficient. For one thing, it costs less over the long run in the building, operation and maintenance of stuff. Also, rejected heat is local, which can be both good and bad, depending if you can use the heat for other things like industrial processes, or heating the home. That gets deep into the weeds and Eli will leave it there


