Dear Judge Alsup: Putting on the Pressure
March 21, 2018
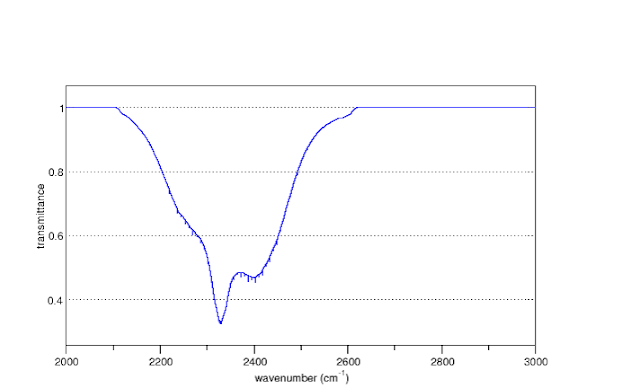
Some may recall that at the end of the first episode, the Spectroscopic Basis, Eli asked why the CO2 IR absorption spectrum at atmospheric pressure
Was different from that at 1/1000 th of an atmosphere
The answer lies in the second letter to the judge, the Quantum Interlude, where Eli discussed how the interaction of light with molecules is really an interaction with the charges, the electrons and nuclei, and how that interaction can be decomposed into a series of multipole moments, the dominant one being the electric dipole, an asymmetry in the distribution of charges. Higher moments, like the quadrupole and shudder, octapole, only become important when the dipole is zero because the molecule is cylindrically symmetric as is the case for N2 and O2.
Comparing the two spectra above, bunnies notice that the baseline has lifted in the first, and if they look real close or blow the figure up, they would see that the absorption lines are wider.
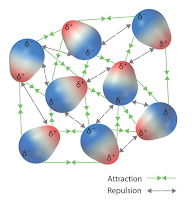 Now those out there who have taken General Chemistry, or even maybe General Physics, can go get a drink while Eli goes on. Turns out that the electrons and nuclei in one molecule or atom can interact with the electrons and nuclei in nearby ones and move the charge around. If we are dealing with molecules that have a dipole moment a picture of what is happening would look like this.
Now those out there who have taken General Chemistry, or even maybe General Physics, can go get a drink while Eli goes on. Turns out that the electrons and nuclei in one molecule or atom can interact with the electrons and nuclei in nearby ones and move the charge around. If we are dealing with molecules that have a dipole moment a picture of what is happening would look like this.
But we need not restrict molecular interactions to only dipole-dipole forces, but can also include the interaction between a dipole and a molecule that has zero dipole moment. In that case, the dipole can interact with the electrons on the dipoleless molecule and shove them around so that there is an induced dipole moment. That is what is happening with the CO2 molecules in the first spectrum. Collisions with N2 or O2 molecules induce a dipole on the N2 and O2 molecules, which then interact via the electric field with each other. This spreads out the spectrum of the CO2 that we observe.
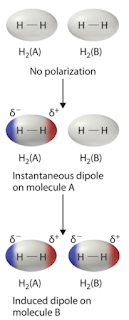 The symmetric N2 and O2 molecules are no longer so symmetric. They can interact with IR light in the regions near their vibrational frequency via the induced electric dipole moment, but wait, there is more. When two molecules with zero electric dipole collide, their electrons and nuclei can also rearrange (as a practical matter it takes a lot less energy in the collision to shove the electrons about than the nuclei, and a lot easier to move the outermost or valance electrons about.
The symmetric N2 and O2 molecules are no longer so symmetric. They can interact with IR light in the regions near their vibrational frequency via the induced electric dipole moment, but wait, there is more. When two molecules with zero electric dipole collide, their electrons and nuclei can also rearrange (as a practical matter it takes a lot less energy in the collision to shove the electrons about than the nuclei, and a lot easier to move the outermost or valance electrons about.
So, let's take a look at what these collision induced dipole moments do to the absorption spectrum of N2 over 10 km at 70% N2. The fuzz is the quadrupole absorption that was shown in the first letter to the judge.
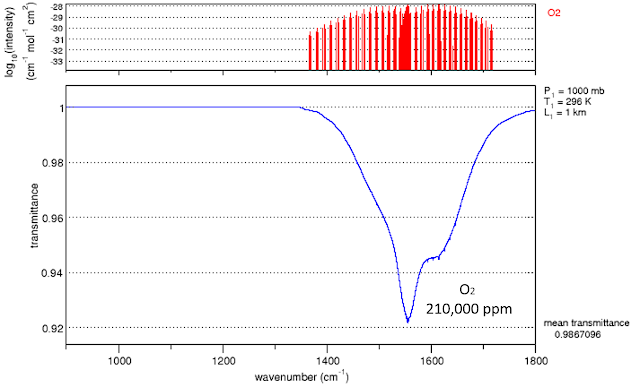
O2, because of it's position at lower frequencies where the 300 K black body spectrum is more intense is perhaps more interesting
and we might better compare it's absorption spectrum with ozone (O3) and methane (CH4) which occur roughly at the same place in the spectrum at their measured mixing ratios in the atmosphere. Even so, the effects of methane and ozone on the absorption are relatively small.
An important point in interpreting these results (Eli's and the KIT group) is that while the concentration of CO2 in the atmosphere has changed from 280 to 410 ppm (see Keeling, Charles) in the last 150 years or more and the concentration of CH4 has more than doubled, the concentration of O2 has changed by a few ppm (see Keeling, Ralph), and N2 bugger all. The small absorptions of O2 and N2 have remained constant only changing really in very deep time.
Eli has written to Dr. Hoepfner about a few questions but has not yet received a reply.
The answer lies in the second letter to the judge, the Quantum Interlude, where Eli discussed how the interaction of light with molecules is really an interaction with the charges, the electrons and nuclei, and how that interaction can be decomposed into a series of multipole moments, the dominant one being the electric dipole, an asymmetry in the distribution of charges. Higher moments, like the quadrupole and shudder, octapole, only become important when the dipole is zero because the molecule is cylindrically symmetric as is the case for N2 and O2.
Comparing the two spectra above, bunnies notice that the baseline has lifted in the first, and if they look real close or blow the figure up, they would see that the absorption lines are wider.
But we need not restrict molecular interactions to only dipole-dipole forces, but can also include the interaction between a dipole and a molecule that has zero dipole moment. In that case, the dipole can interact with the electrons on the dipoleless molecule and shove them around so that there is an induced dipole moment. That is what is happening with the CO2 molecules in the first spectrum. Collisions with N2 or O2 molecules induce a dipole on the N2 and O2 molecules, which then interact via the electric field with each other. This spreads out the spectrum of the CO2 that we observe.
So, let's take a look at what these collision induced dipole moments do to the absorption spectrum of N2 over 10 km at 70% N2. The fuzz is the quadrupole absorption that was shown in the first letter to the judge.
O2, because of it's position at lower frequencies where the 300 K black body spectrum is more intense is perhaps more interesting
The upper scale shows the absorption coefficients of the molecular lines without boadening.
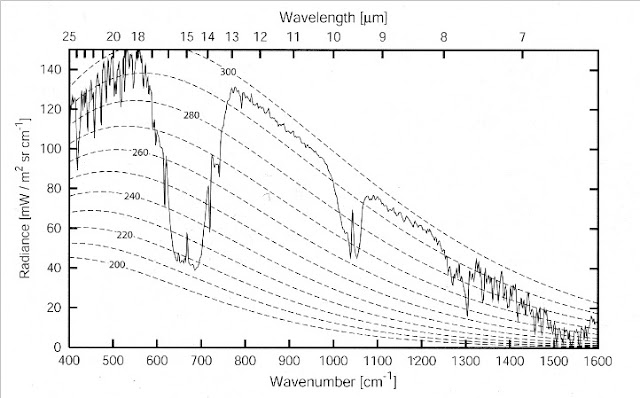
As a final (well semi-final) point, a Rabett could look for the absorption of O2 in the observed high resolution spectrum from the FIRST balloon ~60 km up
Now Eli did say semifinal. Turns out there is a paper by Höpfner, Milz,Buehler,Orphal, and Stiller from the Karlsruhr Institute of Technology that goes through the numbers. They find
The effect of collision-induced absorption by molecularoxygen (O2) and nitrogen (N2) on the outgoing longwaveradiation (OLR) of the Earth’s atmosphere has been quantified. We have found that on global average under clear-sky conditions the OLR is reduced due to O2 by 0.11 W/m2 and due to N2 by 0.17 W/m2. Together this amounts to 15% of the OLR-reduction caused by CH4 at present atmospheric concentrations. Over Antarctica the combined effect of O2 and N2 increases on average to about 38% of CH4 with single values reaching up to 80%. This is explained by less interference of H2O spectral bands on the absorption features of O2 and N2 for dry atmospheric conditions.
Eli has written to Dr. Hoepfner about a few questions but has not yet received a reply.