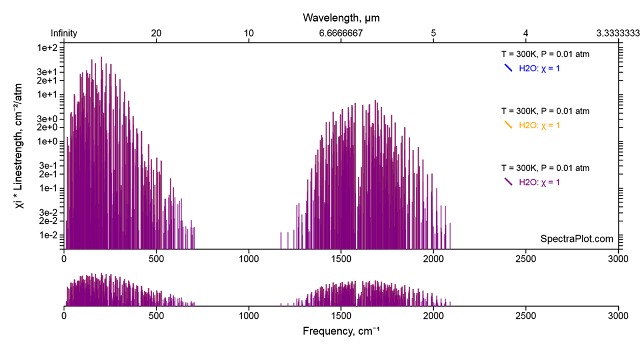Dear Judge Alsup: The Quantum Interlude
March 17, 2018In Part I, the Spectroscopic Basis, Eli looked at measurements of the O2, N2 and CO2 spectra and found that the CO2 is absorption is many times stronger than the O2, and N2 absorption even taking into acount the much higher density of the diatomics . Strong enough that one can neglect the absorption of the other two molecules as a practical matter, however let the Bunny not stop there but go on to the quantum basis of all this trying not to get either too mathematical or too esoteric (esoteric comes in Part III: Putting the Pressure On where the surprises are). Eli will attempt to be correct, but not perfect and certainly not complete, that is a two semester course.
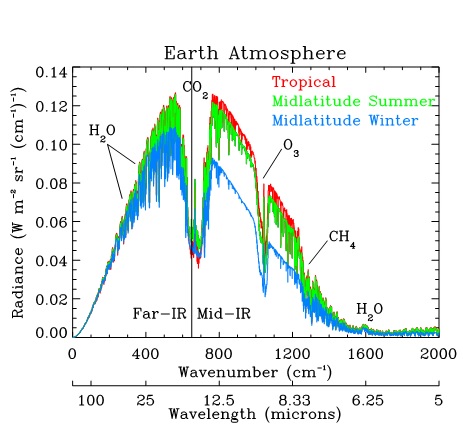
Starting back with neolithic quantum physics, let us now look at the emission spectrum of a blackbody. We can treat the surface of the Earth as one in the IR with unit emissivity (OK ice is a bit different but it is a lot colder so it emits a lot less) and look at the spectrum.we would expect at 290 K
 where your gracious host has marked where our three players, O2, N2 and CO2 would absorb and what the black body emission would be at 290K. The observant among Eli's readers have noticed that there is simply no IR, or darn near none of it out where N2 and the CO2 asymmetric stretch absorbs.
where your gracious host has marked where our three players, O2, N2 and CO2 would absorb and what the black body emission would be at 290K. The observant among Eli's readers have noticed that there is simply no IR, or darn near none of it out where N2 and the CO2 asymmetric stretch absorbs.If you want to know what the bending and symmetric stretching vibrations are, make sure your significant other or keeper is not around. Place your fists on either side of your head and move them up and down or forward and back. Your head is the model of the C atom and the fists are oxygen. That is the bending vibration. There are two such, forward and back and up and down. They have the same frequency and Eli calls them degenerate. If somebunny catches you doing this, you may be so called also. For the asymmetric stretch move one of your fists toward your head and the other away while bending the noggin toward the fist that is trying to hit it. Then reverse. Folks doing this too enthusiastically can knock themselves out.
Rabett Run now needs to crawl a bit further down the physics tree to Electricity and Magnetism. Light (Eli will use the word light to describe IR, which strictly speaking annoys the fussbudgets who reserve light for visible light, but what the heck), is electromagnetic radiation, from the gamma ray to the radio waves and beyond in both directions.
Molecules are composed of atoms, which are composed of positively charged nuclei and negatively charged electrons. The charges have electric fields, which interact with each other and the electromagnetic field. The forces created by the interaction can move the charges relative to each other and in space.
We can describe the field created by the electrons and nuclei one by one or we can describe the potential energy in the field at a point a large distance r from the molecule as a power series in (1/r)n. If r is large, the importance of each term decreases with n. It is pretty well hopeless to describe the field created by the charges one by one especially if they are moving, and the power series converges quickly. That means that each term is a lot bigger than the next so in practice we only need to keep the first non zero term, maybe the second. This power series is called a multipole expansion.
The first term in the multipole expansion is proportional to the sum over all the charges divided by (r). Since molecules are neutral the sum of charges is zero and the first term is zero for a molecule.
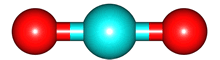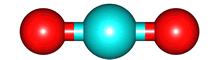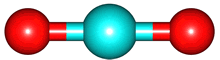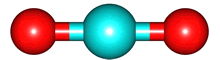
The second term, for which the potential would vary as 1/r2 is called the electric dipole and is equal to Σ qi di where Σ is the sum over all the charges and di is a vector pointing towards charge i. Rather than bothering about the math, let's look at some examples CO2 and H2O.
For the electrons, the image is an electron anomaly distribution, with the blue areas being electron rich and the red electron poor, but the point is that the distribution is also cylindrically symmetric and for every point that contributes positively to to the electric dipole moment there is one that contributes negatively. They cancel, and the net electric dipole moment of ground state CO2 is zero.
The same is true for O2 and N2
The case for H2O is different. The shape of the molecule is bent, there is a region of high electron density on the side of the molecule facing away from the hydrogen atoms and the electron density on the side of the hydrogen atoms facing away from the oxygen atom is electron poor. H2O will have a permanent dipole moment. The blue arrow points towards the region of higher negative charge
When a molecule with a permanent dipole moment rotates (there are three axes that water vapor can rotate about) the dipole moment moves and that movement interacts with light because light is an electro-magnetic field that is influenced by moving dipoles and/or can influence them. By this mechanism rotating water vapor molecules can either gain (absorption) or lose (emission) light (IR or better put Far IR) between 0 and 800 cm-1 accompanied by a change in rotational state. Molecules with zero dipole moment can spin merrily on their way but they do not interact with light in this way. We can see these rotational transition in the spectrum of water vapor between 0 and 800 cm-1
The semi-log plot comes from another handy dandy web app Spectral Plot. The rotational lines (the bunch on the left) overlap the CO2 bending vibration as can be seen in the high resolution FIRST balloon specta taken at about 60 km looking down.
 The bunch of water vapor lines at 1700 cm-1 are the result of the bending vibrational transition. Looking at the electron density map of HOH, it is clear that changing the molecule bends the H-O-H angle will change, vibrating about the equilibrium ground state position as shown in this gif from Marc Henry.
The bunch of water vapor lines at 1700 cm-1 are the result of the bending vibrational transition. Looking at the electron density map of HOH, it is clear that changing the molecule bends the H-O-H angle will change, vibrating about the equilibrium ground state position as shown in this gif from Marc Henry. The instantaneous change in the dipole moment is called the transition dipole moment. It is the non-zero transition dipole moment that makes the bend (Source at UVA)
The instantaneous change in the dipole moment is called the transition dipole moment. It is the non-zero transition dipole moment that makes the bend (Source at UVA)
and asymmetric stretch of CO2 IR active,
while the symmetric stretch is not. A little thought will show that when homonuclear diatomic molecules such as N2 and O2 begin to vibrate there is no change in the dipole moment and therefore they cannot absorb or emit IR accompanied by a change in vibrational state.

A really good analogy to this is a dipole antenna such as the ones used for receiving FM radio.
So how do N2 and O2 interact with light? Well, the next element in the multipole expansion beyond the electric dipole moment is the quadrupole moment. Eli will not bother you with how to calculate it. It turns out that, both N2 and O2 have ferocious quadrupole transition moments, essentially because they are so cylindrically symmetric and the same is true of the symmetric stretch of CO2, but even with a strong quadrupole transition moment the fact that the interaction of a quadrupole with light is proportional to 1/r3 rather than 1/r2 makes their absorption much weaker. There is also an antenna analogy. The Adcock antenna, used for direction finding, is a quadrupole array.
The TL:DR version of this is that it is the interaction of the electromagnetic field of light with the charge distribution of molecules that gives rise to absorption and emission of IR radiation. Although not dealt with here, quantum mechanics tells us about what changes in rotational and vibrational levels are allowed if the electromagnetic interaction is non-zero. If the electric dipole is nonzero, vibrational transitions with unit change in quantum number are strongly favored, the same is true for rotational transitions. Overtones with changes of two or more quanta are very weak.
So stay tuned for the exciting finale.